T5.3 Use case “Secondary data and record linkage”
Background
In epidemiological studies, a vast amount of information has to be gathered on each individual participant, which in turn results in demanding examination protocols and questionnaires. Also, information may not be correctly recalled or not at all known by study participants. It is therefore desirable, to tap external data sources (secondary data) that record objective data and that – if such data are made available – reduce the amount of information to be provided by study participants (primary data). Besides disease registries, most secondary databases store individual level data for other purposes than research as, e.g., claims data collected by health insurances for reimbursement purposes. But these data are nevertheless a powerful resource for research. Making these data accessible for research is a huge challenge because access is restricted by rigid privacy and data protection regulations.
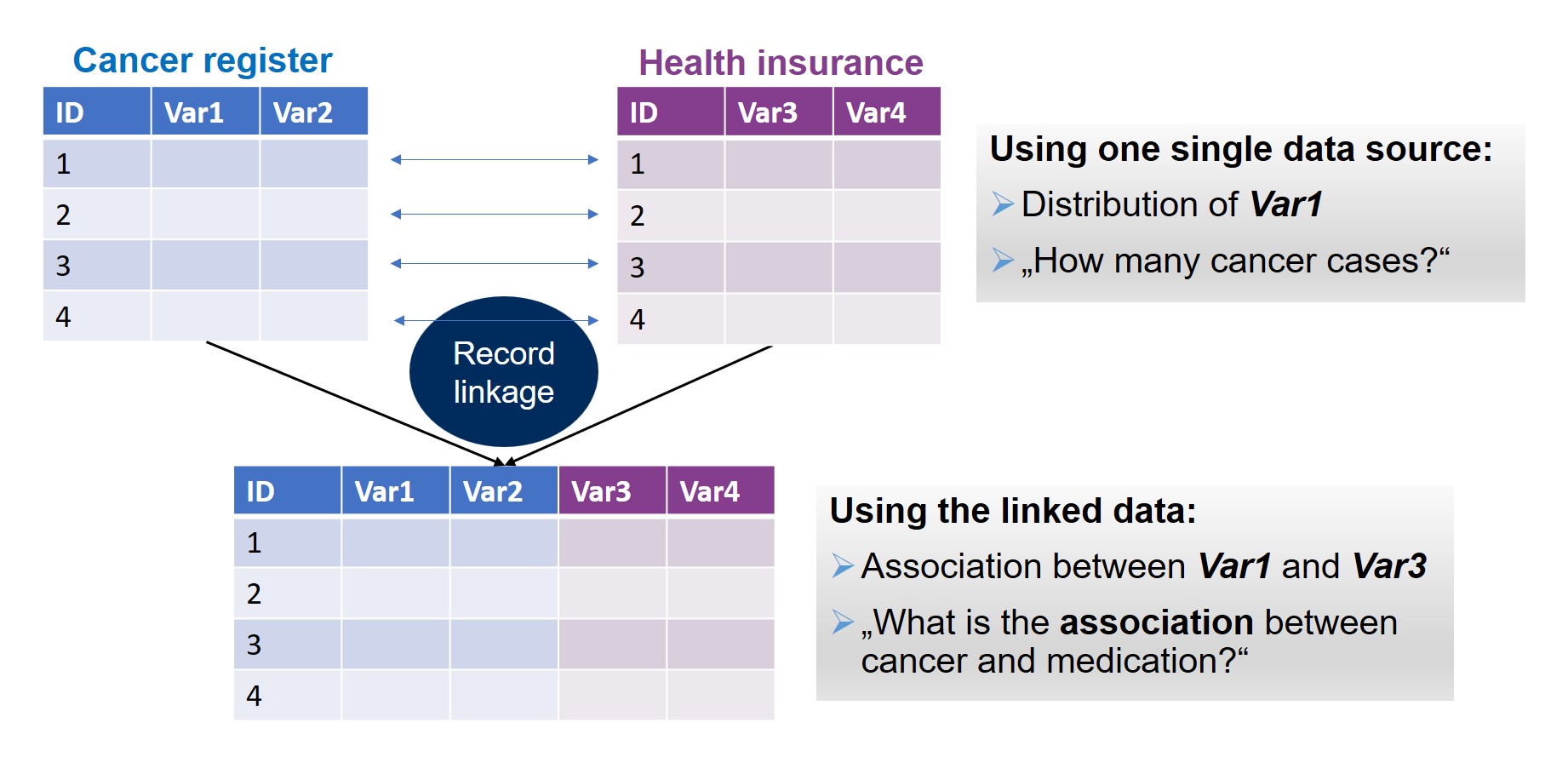
Illustration of research opportunities with linked registry/secondary data.
Record linkage, i.e. linkage of primary data with secondary/registry data sources or linkage of different secondary/registry data sources with each other on an individual level creates synergies. That means, it offers most efficient options for health research where highly informative but yet under-used data are shared and combined to answer research questions that cannot be addressed by traditional single-data-source approaches. These data would also enrich clinical trial data by medical care and outcome data and even by other social data and by this allow for a more holistic approach when, e.g., analysing the efficacy and long-term toxicity of drugs. However, any record linkage is hampered by the fact that no unique identifier exists in Germany as opposed to the Nordic countries like Denmark. Different alternative linkage approaches have been tried in Germany, each having its specific limitations and quality issues like linkage error.
Our Objectives | |||||||
|---|---|---|---|---|---|---|---|
| |||||||
Results & ongoing work
We develop a meta data schema and standards for record linkage and secondary/registry data (in collaboration with TA2 "Standards for FAIR Data" and TA3 “Services”). This includes:
Identification of available and missing standards for the ”Standardisation and interoperability roadmap",
Overview of suitable standards for record linkage and secondary/registry data for “Identification of relevant data formats, standards and terminologies for interoperability” (Read more about data harmonisation),
For the meta data schema and the entry mask of German Central Health Study Hub: Integration of “secondary/registry data” and “record linkage”,
Implementation Guide of the NFDI4Health meta data schema: describing record linkage module of the meta data schema.
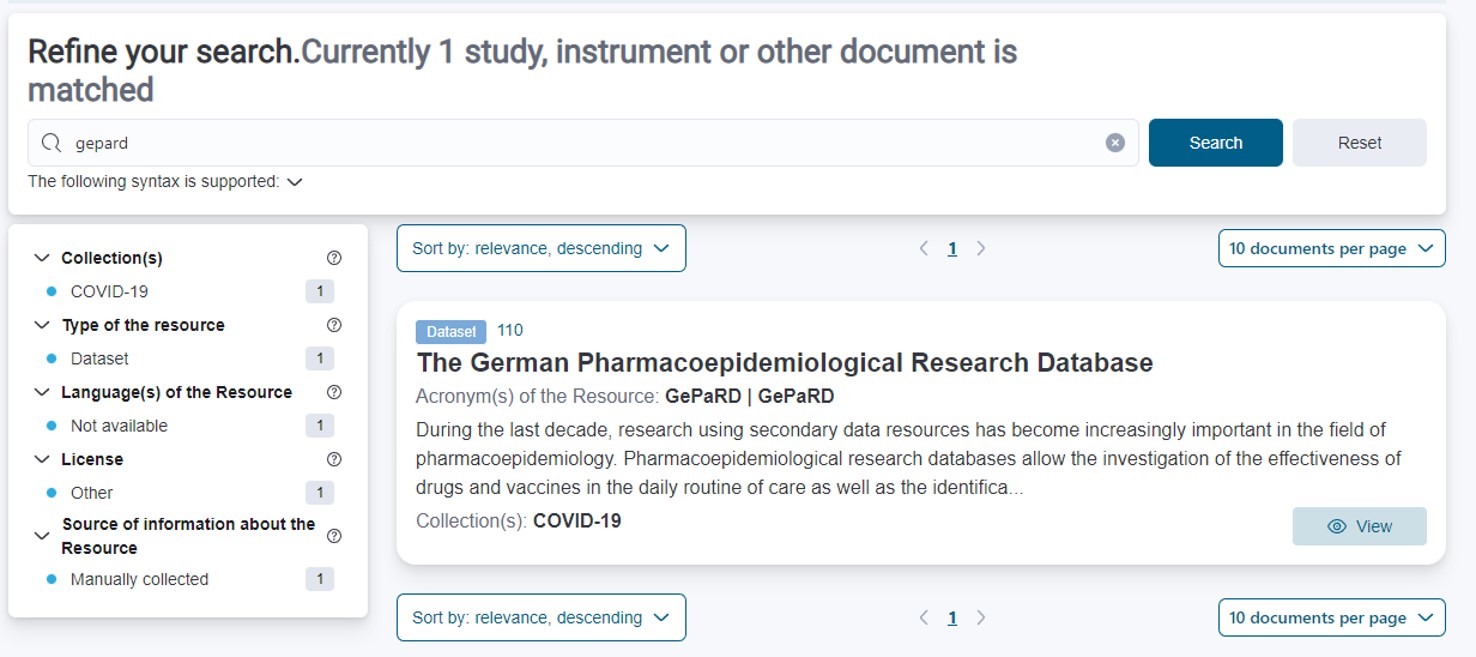
Snapshot of a search for meta data of the secondary data source “GePaRD” on the German Central Health Study Hub.
White paper: Improving record linkage for health research in Germany
In Germany, it is currently difficult to link health data from different sources. This impairs the quality of healthcare - especially in comparison with other European countries. To counter this, NFDI4Health has developed a white paper on record linkage with other experts, including, among others, the Medical Informatics Initiative and the University Medicine Network. The white paper describes the obstacles to the effective use of health data and points to practical and low-bureaucracy solutions that are in line with the European General Data Protection Regulation. It takes an interdisciplinary approach to guide and support scientists for their record linkage projects and gives the following 4 central recommendations to the legislator:
Implementation of a Unique Identifier
Establishment of domain-specific pseudonyms
Establishment of a central oversight and approval authority
Establishment of a decentralised federated research data infrastrcture with central components for record linkage
Full text white paper | Press release white paper | News article white paper
Outreach Activities
Moreover, we organized several events and hold talks related to record linkage and the white paper to involve the community and offer support:
More than 20 talks on the status quo & our political demands
Some Examples
More than 20 talks on the status quo & our political demands
Several workshops to get input from experts for the white paper
Some Examples
Provision of an annotated template of an informed consent form for record linkage
Informed Consent Form
Provision of an annotated template of an informed consent form for record linkage
The white paper, our outreach activities and the annotated template of an informed consent form were developed in cooperation with TA6 “Privacy & Data Access in Concert”.
Publications
Abaza H, Shutsko A, Golebiewski M, Klopfenstein S, Schmidt CO, Vorisek CN, Brünings-Kuppe C, Clemens V, Darms J, Hanß S, Intemann T, Jannasch F, Kasbohm E, Lindstädt B, Löbe M, Orban E, Perrar I, Peters M, Sax U, Schulze MB, Schupp C, Schwarz F, Schwedhelm C, Strathmann S, Waltemath D, Wünsche H, Zeleke A. The NFDI4Health metadata schema (V3_3). Köln 2023. https://repository.publisso.de/resource/frl%3A6472531
Intemann T, Kaulke K, Kipker D-K, Lettieri V, Stallmann C, Schmidt CO, Geidel L, Bialke M, Hampf C, Stahl D, Lablans M, Rohde F, Franke M, Kraywinkel K, Kieschke J, Bartholomäus S, Näher A-F, Tremper G, Lambarki M, March S, Prasser F, Haber AC, Drepper J, Schlünder I, Kirsten T, Pigeot I, Sax U, Buchner B, Ahrens W & Semler S. White Paper: Verbesserung des Record Linkage für die Gesundheitsforschung in Deutschland. 2023. https://doi.org/10.4126/FRL01-006461895
Intemann T, Lettieri V, Kipker DK, Kuntz A, Ahrens W, Pigeot I, Buchner B, Sax U, Fluck J, Fröhlich H, Geok Ng H, Hahn H, Kirsten T, Lange JJ, Lassen-Schmidt B, Prause G, Richter A, Schmidt C. Informed Consent zum Record Linkage: Best Practice und Mustertexte. Publisso, 2023. https://doi.org/10.4126/FRL01-006399943
Pigeot I, Fröhlich H, Intemann T, Prause G, Wright MN. KI und die Nationale Forschungsdateninfrastruktur für personenbezogene Gesundheitsdaten (NFDI4Health). In: Dössel OS, Tobias; Rutert, Britta, editor. Künstliche Intelligenz in der Medizin. Denkanstöße aus der Akademie: Eine Schriftenreihe der Berlin-Brandenburgischen Akademie der Wissenschaften, Nr. 11. Berlin: Berlin-Brandenburgische Akademie der Wissenschaften; 2023. p. 62-74.
Pigeot I, Intemann T, Kollhorst B, Sax U, Ahrens W. FAIRifizierung von Real World Data für die Gesundheitsforschung: Ein Petitum für modernes Record Linkage. Prävention und Gesundheitsförderung. Präv Gesundheitsf (2022). https://link.springer.com/article/10.1007/s11553-022-00973-x
Contact

Prof. Dr. Iris Pigeot
Measure-Lead: T5.3 “Use case Secondary data and record linkage”
E-Mail: pigeot@leibniz-bips.de
Phone: +49 (0)228 73-60351
Leibniz Institute for Prevention Research and Epidemiology – BIPS GmbH
Achterstraße 30
D-28359 Bremen

Prof. Dr. Wolfgang Ahrens
Measure-Lead T5.3 “Use case Secondary data and record linkage”
E-Mail: ahrens@leibniz-bips.de
Phone: +49 (0)421 218-56822
Leibniz Institute for Prevention Research and Epidemiology – BIPS GmbH
Achterstraße 30
D-28359 Bremen
 English
English  Deutsch
Deutsch 